The 501 Blues: How worried should we be about novel SARS-2 variants?
January 18/2021
By Dr. David Fisman, Epidemiologist and Professor at DLSPH
Over the Christmas break news began to circulate of a novel SARS-2 variant, which had appeared in southern England in September 2021 and which has rapidly become the dominant strain of SARS-2 across England. Particularly worrisome is the fact that this new strain of SARS-2 has a mutation known as N501Y, which is known to allow the virus to “bind better” to ACE-2, the chemical that SARS-2 uses to infect human hosts. The new lineage is known by a few different names, including the “B.1.1.7” lineage, and “VOC 202012/01” (meaning “variant of concern, year 2020, month 12, variant 1”) by the World Health Organization. A few days later, South Africa reported that another variant with the N501Y mutation that also seems to be more infectious and which seems to have spurred a summertime resurgence of COVID-19 in South Africa. This week a third such variant was reported in Brazil, which is also experiencing a summertime resurgence (it’s known as “P.1.”). A fourth such variant has been reported in Columbus, Ohio. What’s going on?
If you want to take a deep dive into questions related to variants, I would highly recommend an excellent long piece by journalist Kai Kupferschmidt in Science that you can find here. Genetic epidemiologist Bill Hanage of Harvard School of Public Health has also written excellent threads about variants that you can find on Twitter. Formal reports on B.1.1.7. and P.1. are available here and here. Colleagues at Imperial College in London have done a detailed epidemiological analysis on B.1.1.7. and have found convincing evidence that it is more infectious than previously circulating strains, and also seems to be the dominant strain in kids in England now.
If you don’t want to take a deep dive into the world of mutations, variants and strains, here are a few key points to keep in mind:
- Mutation of a new (in humans) virus is expected. Viruses mutate (change) all the time, and it is expected that this virus would become better and better adapted to humans as time goes on. For these new N501Y-mutants to “take over” in populations that already have widespread SARS-2 transmission, like in England and Brazil, means that this virus must have a competitive advantage over other strains. The fact that many different variants are emerging, around the world, with the N501Y mutation suggests that these viruses are “finding a solution” to the problem of how to infect humans more efficiently, something called “convergent evolution”.
- The fact that these new strains are more infectious doesn’t mean they are more deadly. Indeed, we can probably expect SARS-2 to become gradually less deadly over time, as milder, longer, infections probably allow a virus to create more new infections than would occur if someone was severely sickened. This phenomenon (movement towards viral strains that make people less sick, over time) is called “balanced pathogenicity”.
- An important question is whether new variants might be less likely to be prevented by our vaccines than existing circulating strains. So far it doesn’t look like that will be the case. A key advantage of new mRNA-based vaccines is that they give your body a “recipe” to create antibody against the virus’ spike protein; when that protein mutates, the vaccine manufacturers can change the “recipe” for the vaccine in around 6 weeks, much faster than would be possible with traditional vaccines.
What can we do about these new variants? Firstly, knowing that our existing vaccines are probably effective against variants, we should try to get our population vaccinated as fast as we can, with the vaccine we do have. We should be prepared for the possibility that, hard as it has been to control COVID-19 to date, it may become harder still if these variants become common.
The graphs below, from our colleagues at the Canadian company Scarsin, forecast epidemic curves with and without vaccination, and under varying assumptions about how much B.1.1.7. is circulating in Canada. Having these strains circulate widely will be a setback for epidemic control here. Orange, blue and gray curves represent increasing fractions of B.1.1.7.; black and purple curves represent our current trajectory with (purple) and without (black) vaccine.
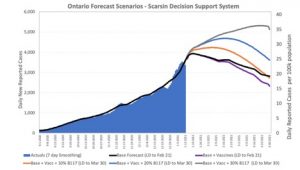
Ontario Forecast Scenarios – Scarsin Decision Support System
That may mean that our epidemic is longer, and larger, than it would have been without these variants. That news comes at a time when Canadian ICUs are already quite full, and starting to prepare for extreme scenarios where healthcare workers need to decide who can, and cannot, have access to a ventilator for lifesaving care. We may want to consider restricting non-essential travel in and out of Canada; some restrictions on flights from the United Kingdom were implemented after B.1.1.7. emerged, and yesterday the United Kingdom, in turn, imposed travel restrictions on S. America and Portugal due to 501.v2. The importance of limiting travel when new variants and strains emerge, at least until they can be better characterized, is laid out here in a Twitter thread by complex systems scholar Yaneer Bar-Yam. Lastly, we need to build capacity for Canadian labs to do “phylogenetics” and “deep sequencing”, which would allow us to identify new variants and figure out how they have evolved.
If we don’t have the ability to do such sequencing rapidly in Canada, how will we know whether novel variants are here? We can continue to pay close attention to our epidemiological data, and look for important changes. We may see some shifts in our epidemiology here in Ontario now, as the percentage of tests positive for SARS-2 in kids appears to have risen sharply over the holiday (as shown in the figure below; red and orange curves are kids under 10, and teens, respectively). Whether this reflects changes in the virus, or changes in behavior (such as Christmas gatherings) is unclear right now, but we may be able to answer this question in the weeks ahead.
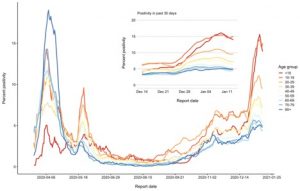
Percentage of tests positive for SARS-2 in kids.